Technical Challenge
Oxygen transport is a key factor in the design of cell culture systems such as organs-on-a-chip, microphysiological systems, and bioreactors. In some cases, prescribed gradients in oxygen concentration are desired (for example, to promote cell function zonation); in other cases, uniform concentration distributions are preferred. Oxygen distribution in a microchannel is determined by channel geometry, flow rate, and cell metabolism kinetics. Understanding the effects of these features on oxygen transport is essential for designing effective cell culture systems and bioreactors.
Controlling the shear stress on cells is also an important design feature in many flow-based cell culture systems. Cell morphology and viability may be affected by shear stress, which strongly depends on channel geometry and flow rate. Depending on cell type, there may be an upper bound, a lower bound, or a range of desired or acceptable shear stresses.
Veryst Solution
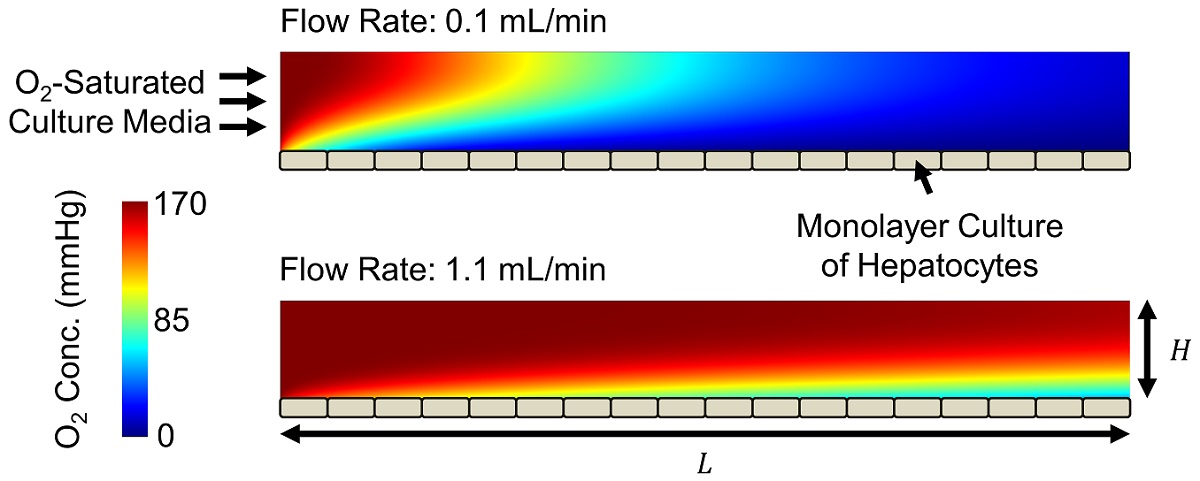
Veryst developed a computational fluid dynamics (CFD) multiphysics model to quantify oxygen transport and uptake as well as shear stresses in a microchannel with a monolayer culture of hepatocytes (Figure 1). We varied the channel height H between 0.1 and 1.0 mm and the fluid flow rate Q between 0.1 and 2.0 mL/min. We assumed that the culture media entering the channel is saturated with oxygen. We modeled oxygen uptake by the hepatocytes with Michaelis-Menten reaction kinetics based on published parameter values. The Peclet number [the ratio of oxygen transport by advection (the flow) to that by diffusion] is 400 for the largest channel height / flow rate combination and 40 for the smallest. This demonstrates that oxygen transport occurs primarily by advection.
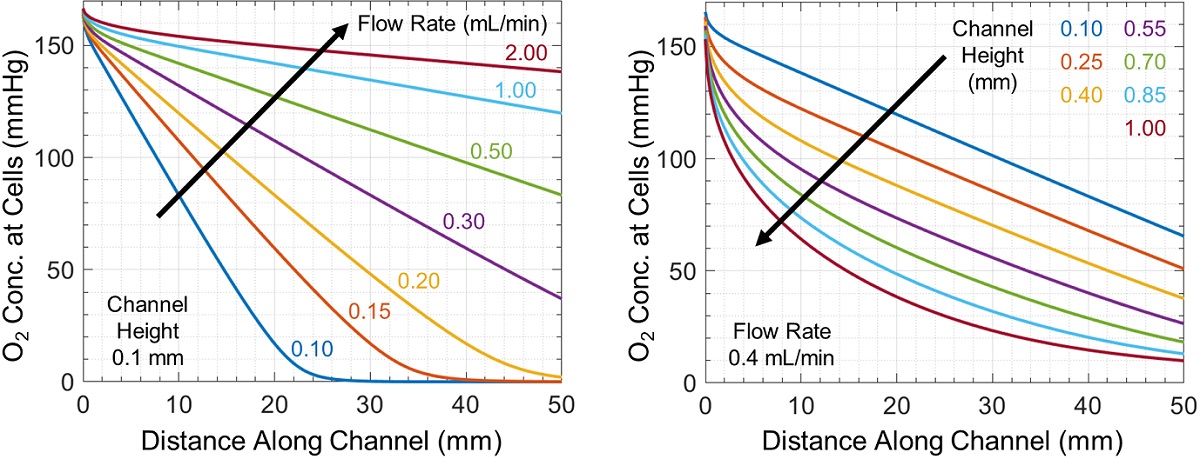
Oxygen is depleted throughout the microchannel by cellular uptake, producing the horizontal and vertical concentration gradients shown in Figure 1. The oxygen concentration experienced by cells changes throughout the channel and is controlled by flow rate and channel geometry (Figure 2). Increasing channel height or decreasing flow rate produces larger concentration gradients, in some cases fully depleting oxygen at or before the end of the channel.
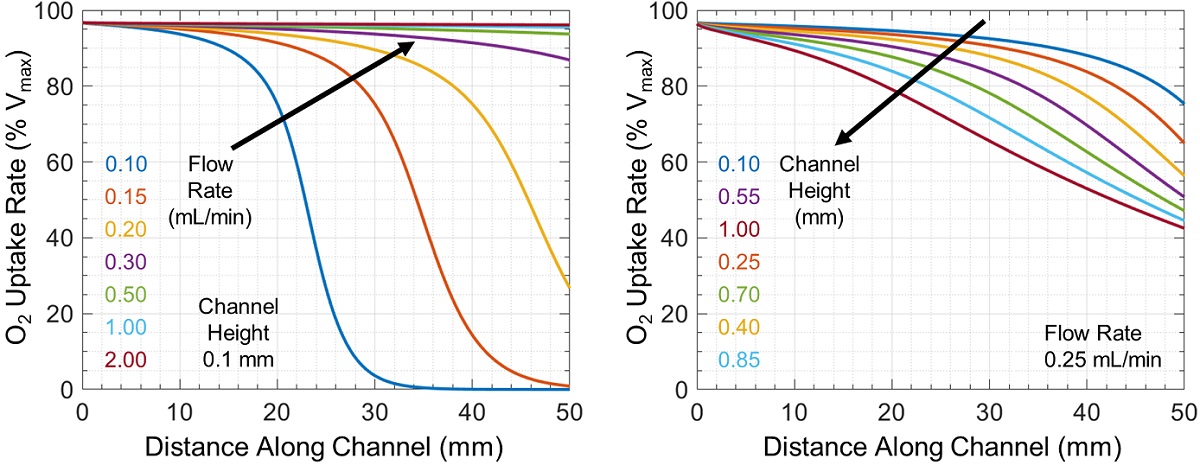
The associated rate of cellular oxygen uptake relative to the maximum possible uptake rate Vmax is shown in Figure 3. In some cases, there is a large difference in oxygen uptake between cells near the channel inlet and outlet (which can change either sharply or uniformly throughout the channel), while in other cases all cells have similar uptake rates.
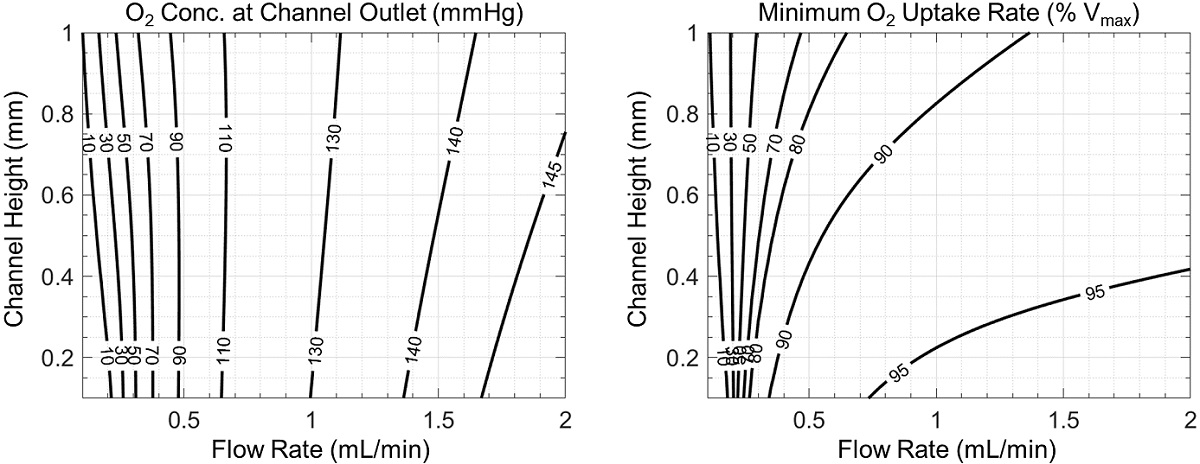
Spatial variation in cellular exposure to oxygen or other metabolites and/or signaling molecules is known as zonation and can produce profound differences in cell function. We show two measures of zonation in the microchannel in Figure 4: the average oxygen concentration at the microchannel outlet (an empirically observable quantity) and the minimum cellular oxygen uptake rate in the microchannel (which is directly measurable in simulations but must be inferred in experiments). In each case in Figure 4, smaller values indicate greater potential differences in cell function at the inlet and outlet of the channel, and therefore increased zonation.
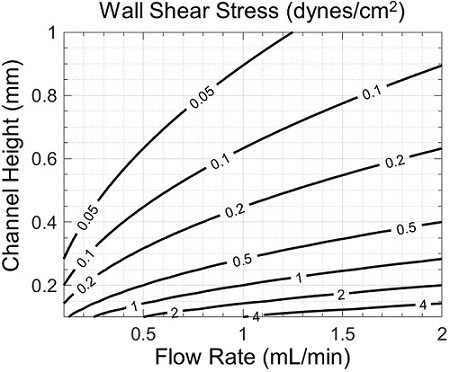
Cell morphology and viability may also be affected by shear stress, which strongly depends on channel geometry and flow rate (Figure 5). Though fast flows in shallow channels are associated with lower variations in oxygen concentration (Figure 4), they also produce higher shear stresses (Figure 5). Therefore, a tradeoff may exist between desired zonation and shear stress, which may be properly assessed with the help of simulation.
While we have specifically considered oxygen in this study, the same approach may be used to investigate the transport, exchange, and interaction of drugs, growth factors, other soluble gases such as carbon dioxide, nutrients, and waste products of cellular metabolism. Furthermore, such models may be extended beyond monolayer cell cultures, for instance to porous or multilayer cultures, where fluid flow and species transport in the culture and cell-free regions are considered. Computational models may also be used to infer unknown kinetic and transport properties from experimental data, which may then be used in other models to optimize system performance.
Conclusion
Veryst evaluated oxygen transport and consumption in a microchannel bioreactor containing hepatocytes using CFD multiphysics simulation to determine cellular oxygen uptake rate, zonation, and shear stress.
Simulations such as these may be used to design and optimize the performance of cell culture systems such as organs-on-a-chip, microphysiological systems, and bioreactors.