Biora Therapeutics is developing the BioJet™ oral delivery platform, a swallowable smart capsule that uses needle-free liquid jet injection to precisely deliver large-molecule drugs to the intestinal mucosa. Biora partnered with Veryst to simulate and optimize device activation, jet formation, and injection performance—accelerating design cycles, reducing development risks, and shortening the path to clinical trials.
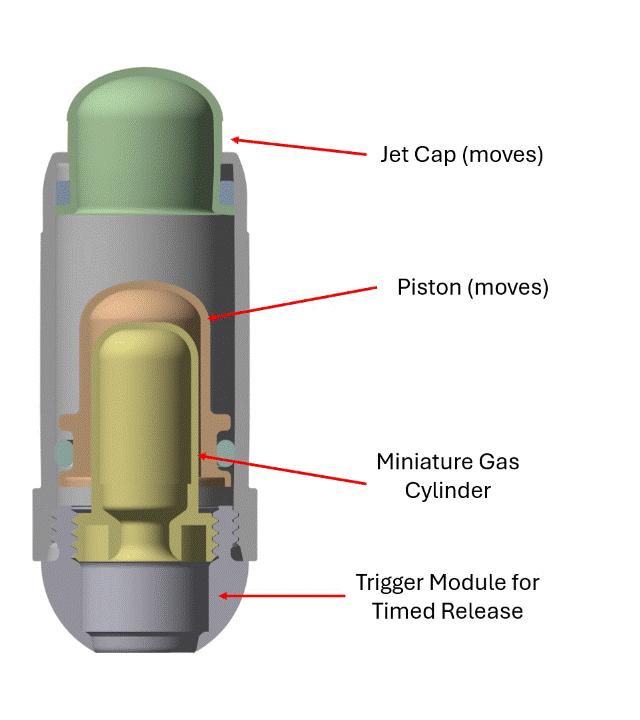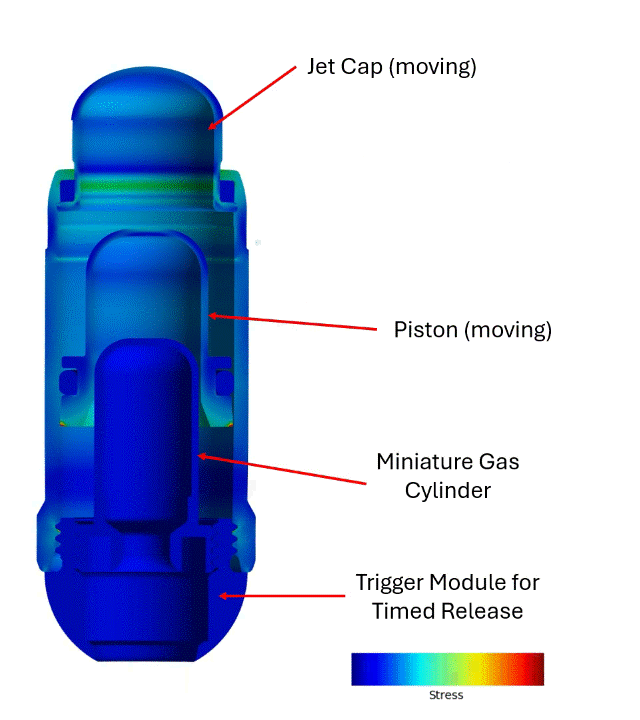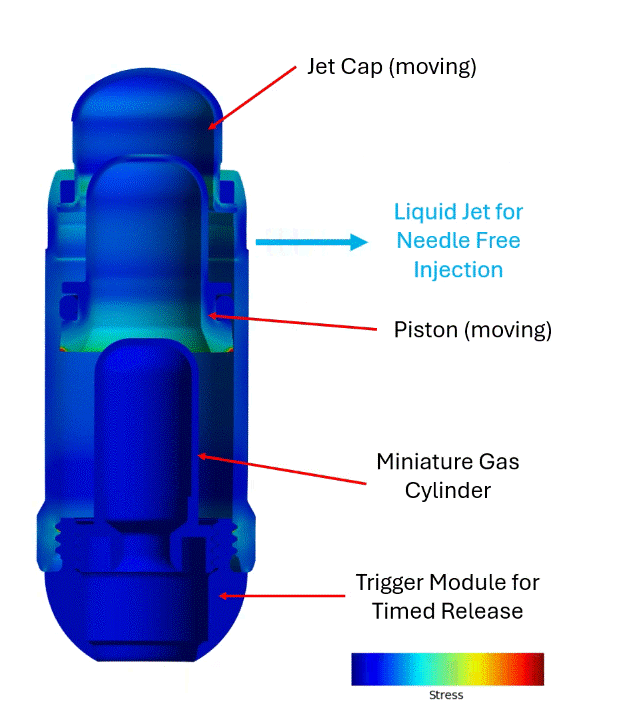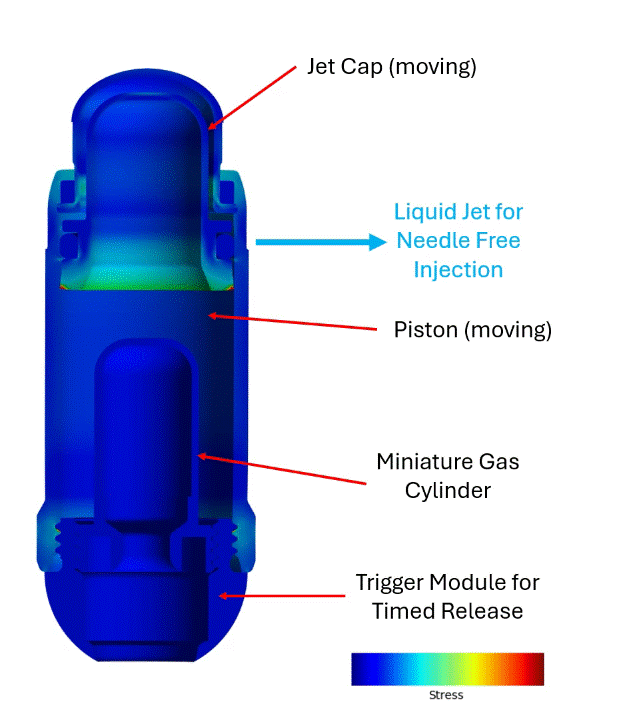
The BioJet device uses a unique pressurized gas cylinder (Figure 1) to power the device and create high-speed liquid jets of drug solution for a needle-free injection. During transit to the small intestine, the device is inert. Upon arrival in the small intestine, a pH sensitive trigger dissolves, allowing the pressure in the gas cylinder to drive the piston. The drug is contained between the piston and the jet cap, and the pressure on the piston forces the jet cap to open, exposing small holes for jetting.
The engagement with Veryst began with early BioJet device prototypes and bench test data. Veryst first created and validated rapid design tools based on fundamental jet nozzle and fluid mechanics theory, streamlining Biora’s engineering decisions. Then, Veryst built a detailed finite element model to simulate activation of the BioJet device and the fluid pressure that forms and drives the jets. The model included the rapid gas expansion driving the piston; frictional forces between the internal components that drive losses in the system; and momentum and inertia of the moving parts. The model enabled the Veryst and Biora teams to predict how design changes would impact the formation and force of the jets, which are critical performance indicators of the system. Example simulation output is shown in Figure 1.
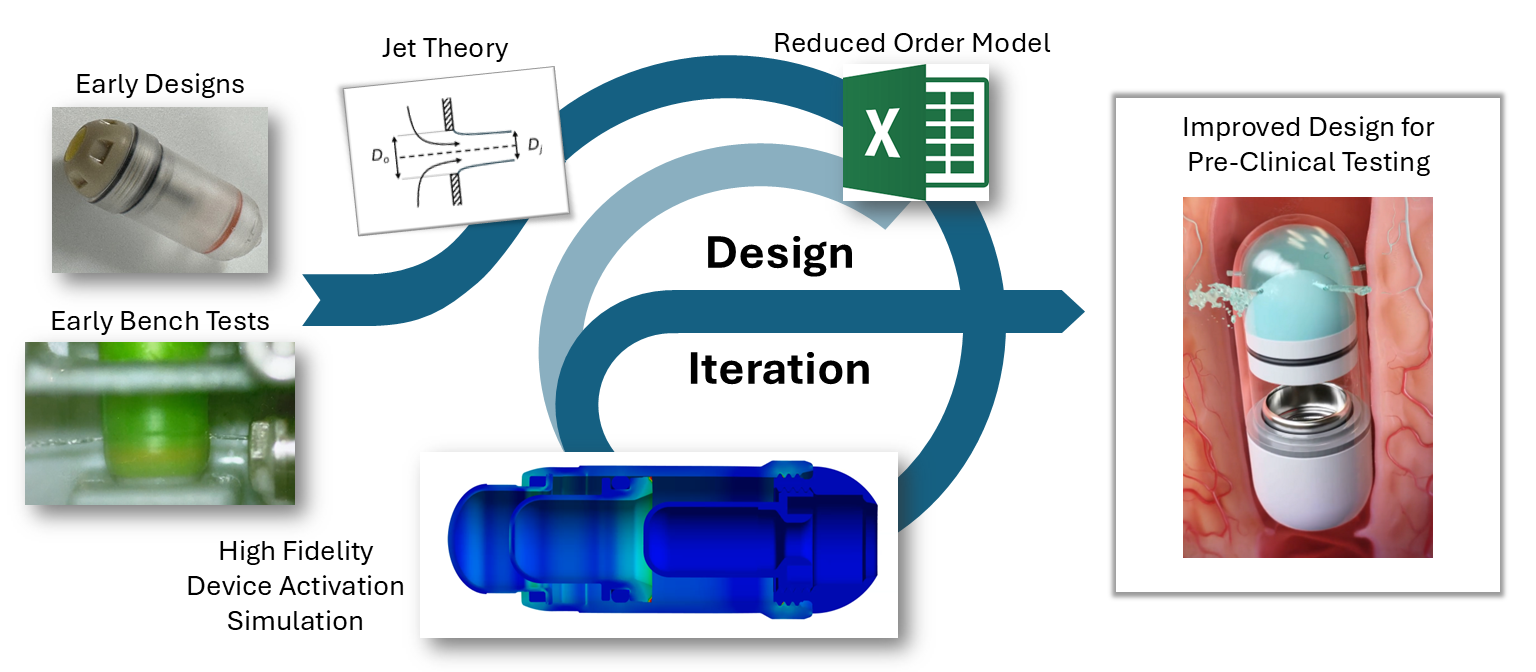
Veryst also performed Computational Fluid Dynamics (CFD) analyses to inform and optimize jetting and jet force for a range of design, operating, and drug parameters. The team’s multidisciplinary approach—combining fluid mechanics, structural analysis, and rapid prototyping—provided clear, real-time design insights and accelerated learning and design iteration (Figure 2).
This collaboration enabled Biora to meet aggressive R&D milestones and deliver devices ready for preclinical trials. The work exemplifies how expert engineering and innovation can advance transformative combination products, with the potential to replace traditional needle-based injections, improve patient comfort, and expand therapeutic access.
Biora TherapeuticsTM and BioJetTM are trademarks of BT Bidco Inc., dba Biora Therapeutics.