A key challenge in drug product development is its compatibility with the delivery system. Drug product formulators need early guidance on drug-device compatibility to ensure the correct delivery system is chosen before finalizing the formulation and proceeding to pre-clinical trials. To address this challenge, Merck and Veryst partnered to create a reduced-order model and analysis tool for spring-driven dual chamber autoinjectors (AIDCs) to accelerate drug-device compatibility testing and drug product development. Veryst incorporated key phenomena including the motion of two stoppers, air compression, solution flow through the needle and bypass channel, and empirically-measured stopper friction dynamics. The modeling framework allows the end-user to modify the device design parameters, formulation properties, needle geometry, and spring characteristics to predict essential performance requirements (EPRs) such as injection time, stopper trajectories, and maximum allowable diluent volume.
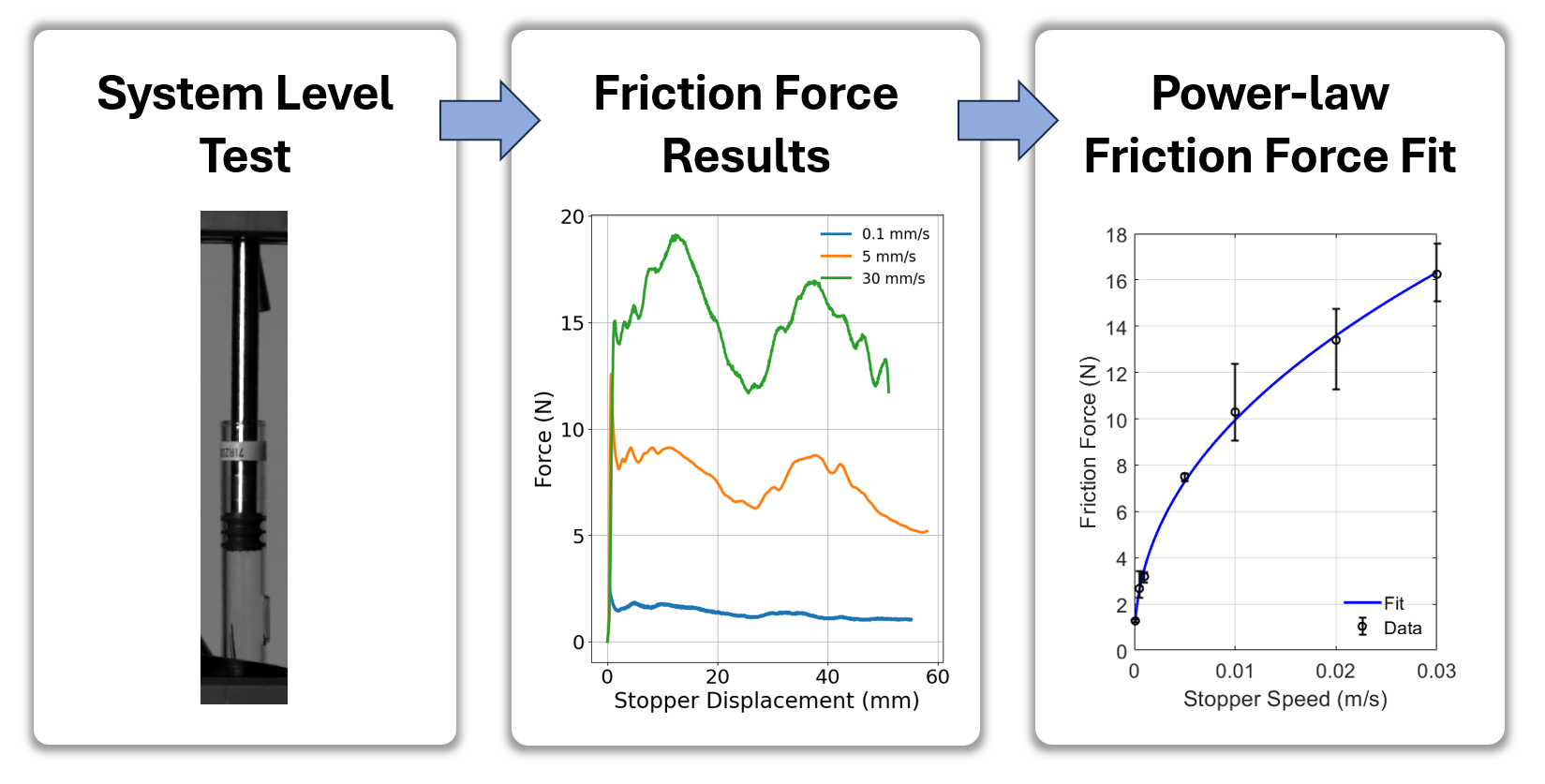
The modeling framework developed by Veryst and Merck partitions AIDC operation into distinct physical stages—pre-reconstitution, priming, and injection—each governed by its own set of equations of motion. The equations of motion include the effects of needle flow resistance via the Hagen-Poiseuille law, linear spring force via Hooke’s law, chamber air compression via the ideal gas law, and an experimentally derived stopper friction vs. glide speed relationship. We modeled the friction between the stopper and syringe based on laboratory test data (Figure 2) and an empirical model fit. We included the effect of back-pressure during subcutaneous injection with a simplified empirical model based on literature data.
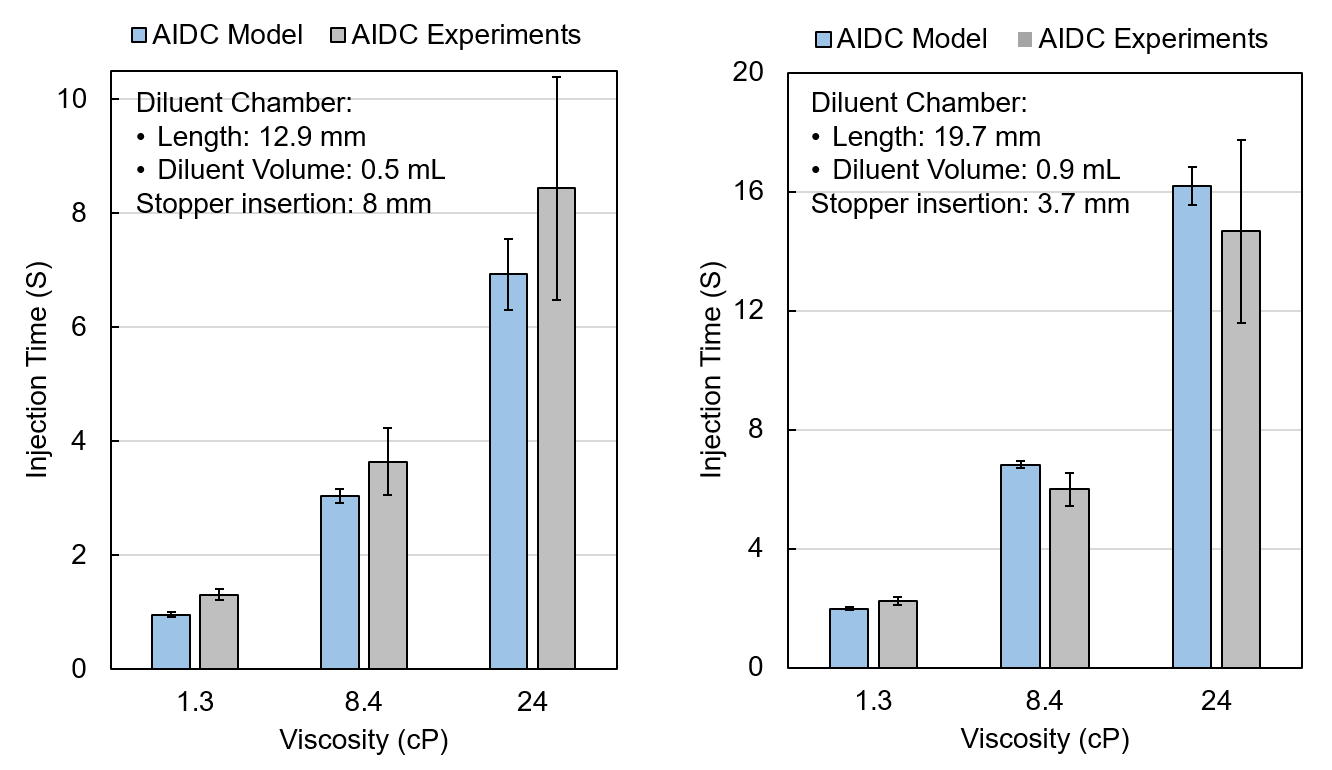
The team validated the model with experimental injection time data, demonstrating strong agreement across a range of diluent volumes, solution viscosities, and initial stopper positions (Figure 3). The model additionally captures how performance varies with formulation viscosity, needle gauge, and spring force.
The AIDC modeling framework enables drug development scientists and formulators to rapidly perform “virtual prototyping” of new drug-device combinations, without the need for extensive bench testing, accelerating technical due diligence and trade-off assessments (e.g., needle size, diluent volume). The modeling framework strengthens the robustness of AIDC performance across the drug delivery landscape—especially for lyophilized and sensitive biologic therapies.
The results are published in Drug Delivery and Translational Research.
Sahab Babaee, Matthew Hancock, Joseph Barakat, Brandon Vuong, Kavin Kowsari, Sean Teller, Lynn Lu, Adriel Gonzalez, Steven Persak, Wail Rasheed. A modeling framework for spring-driven autoinjectors with dual-chamber cartridge. Drug Delivery and Translational Research, July 2025. https://doi.org/10.1007/s13346-025-01898-6.