Technical Challenge
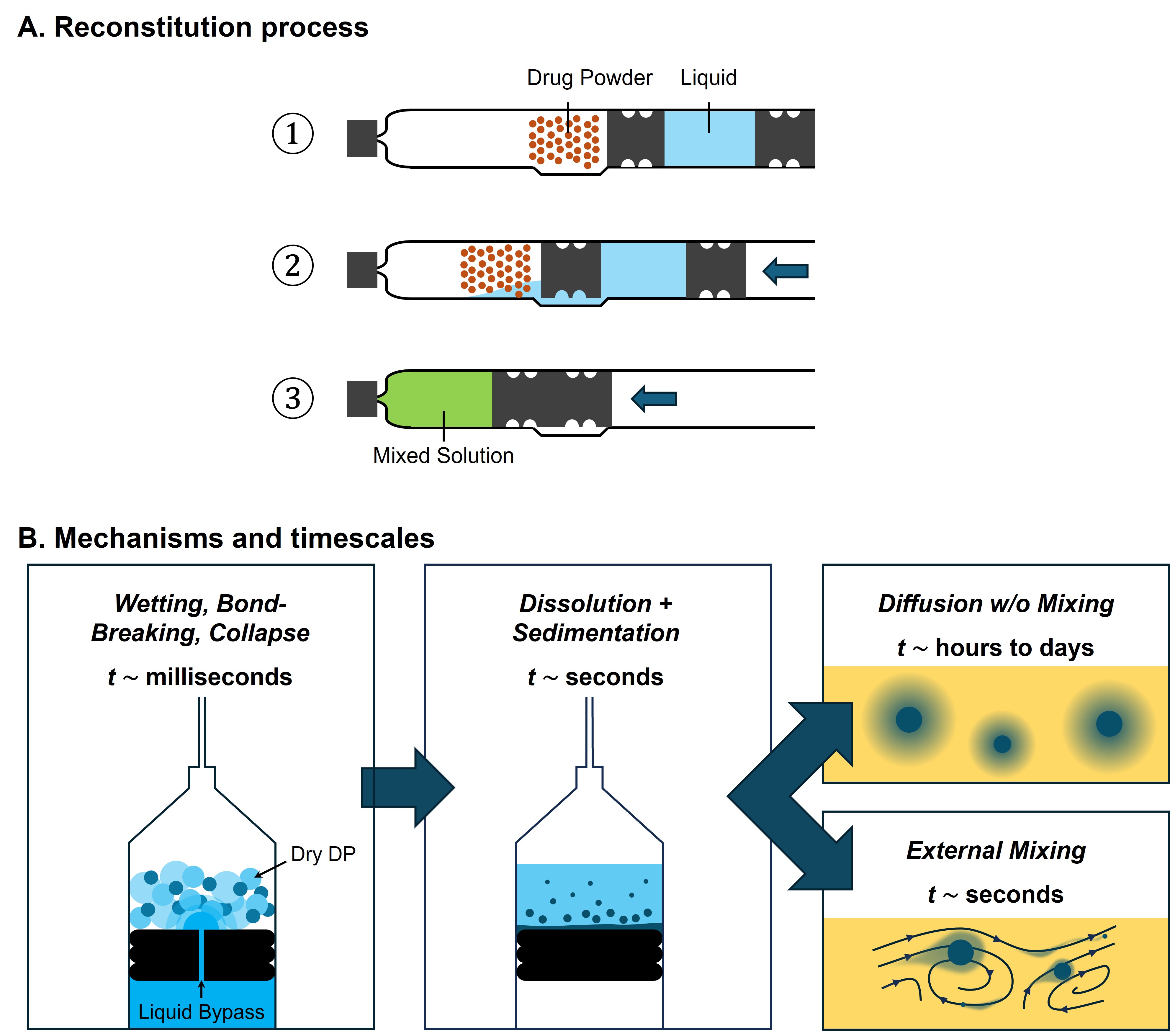
Freeze-dried (lyophilized) drug products extend shelf life and ensure drug stability for combination products such as autoinjectors and pre-filled syringes. A critical pre-injection step is the rapid and complete reconstitution of the freeze-dried drug product (DP) (Figure 1), achieved by diluent flowing into a mixing chamber to dissolve the DP (Figure 2A). Efficient reconstitution is vital for dose accuracy, therapeutic effectiveness, patient safety, and user convenience. DP dissolution can be rate-limiting, especially when active mixing is required to achieve a uniform solution (Figure 2B). Here, we focus on simulating the dissolution kinetics and their dependence on diluent volume, DP concentration, solution viscosity, and DP particle size distribution.
Veryst Solution
Veryst used multiphysics simulation to model the DP dissolution process, employing laminar flow and species transport physics to model solubilized DP distribution. The evolution of DP particle size was captured using a custom Population Balance Equation, where dissolving particles acted as sources within the species transport framework. Model inputs included initial DP particle size distribution, total DP mass, and diluent viscosity and volume.
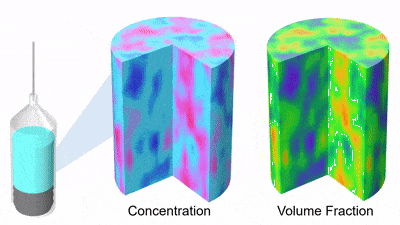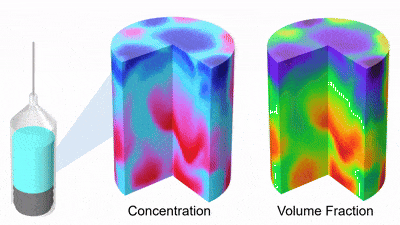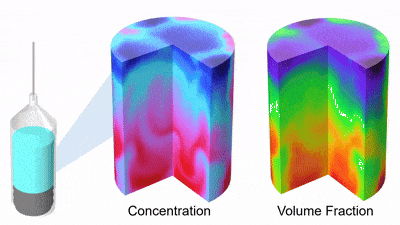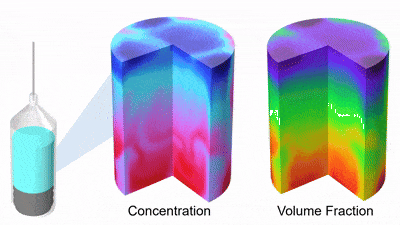
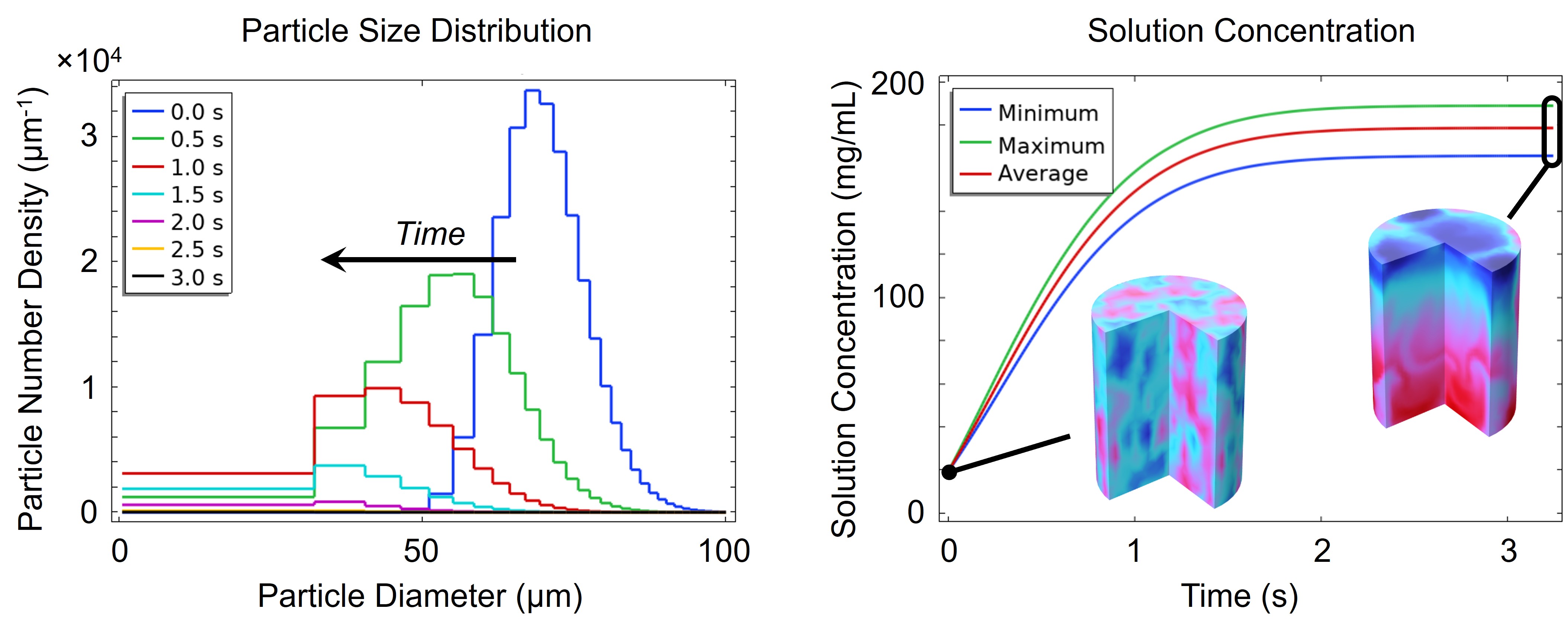
As DP particles dissolve, their size distribution shifts to smaller diameters and broadens, while solution concentration rises (Figures 3 and 4). Gravity-driven particle sedimentation induces a vertical concentration gradient (Figure 4). Consequently, active mixing is typically needed to achieve rapid and homogeneous reconstitution.
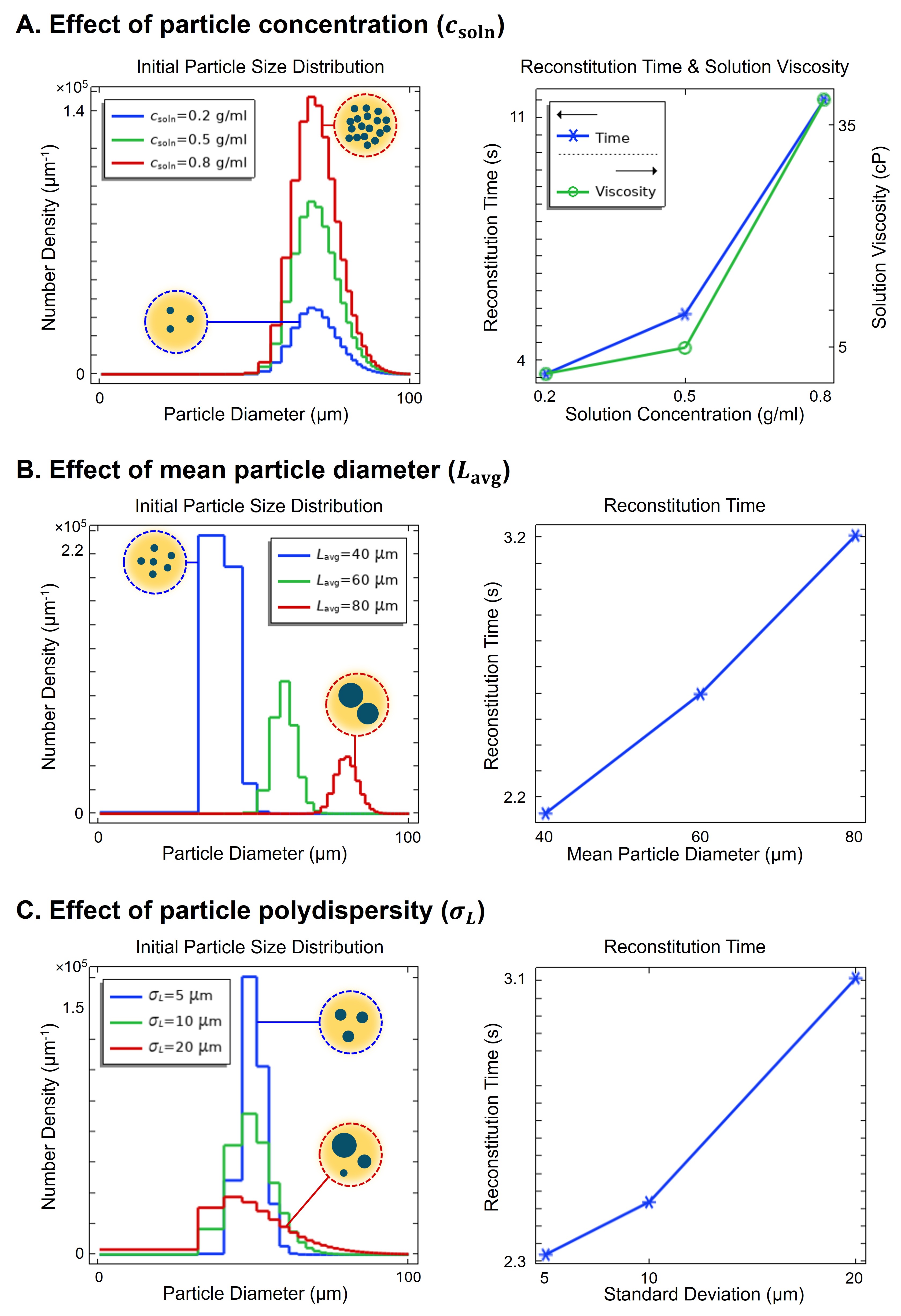
Parametric analysis (Figure 5) highlights key insights: higher DP particle concentrations increase solution viscosity and extend reconstitution times (Figure 5A); larger mean particle size slows dissolution (Figure 5B); and increased polydispersity further delays reconstitution as larger particles dissolve more slowly (Figure 5C).
Conclusion
Veryst used CFD simulations of freeze-dried drug product reconstitution to visualize the complex, transient, and coupled flow and drug product particle dissolution, a challenging task to achieve experimentally due to the often-self-contained nature of combination products and the short time scales of reconstitution. Simulations such as these enable alternate designs, operating protocols, formulations, and material and fluid properties to be evaluated quickly, providing insight, and expediting autoinjector and formulation development. Our results provide insights to ensure rapid and effective reconstitution and reliable combination product performance.