Technical Challenge
Manufacturing diagnostic kits often involves the drying of reagent solutions to prepare the kit for shelf storage. Later, during use, the dried-down reagents are reconstituted into sample or buffer solutions and used for disease diagnostics. During manufacturing, the drying rate depends on a range of environmental and process parameters, including the relative humidity in the drying oven, the air temperature, and the extent and distribution of air circulation.
Accurately predicting the time needed to evaporate a small (microliter) volume of fluid requires modeling several coupled physical processes. For all but the simplest geometries, such models generally require advanced numerical simulation.
Veryst Solution
Veryst used COMSOL Multiphysics to simulate reagent dry-down in a microwell initially filled with 5 μl of solution. The model accounts for coupled fluid flow, vapor transport, latent heat, free-surface motion, and substrate wettability. We varied the relative humidity and setpoint temperature of the drying oven to determine the resulting impact on the evaporation rate.
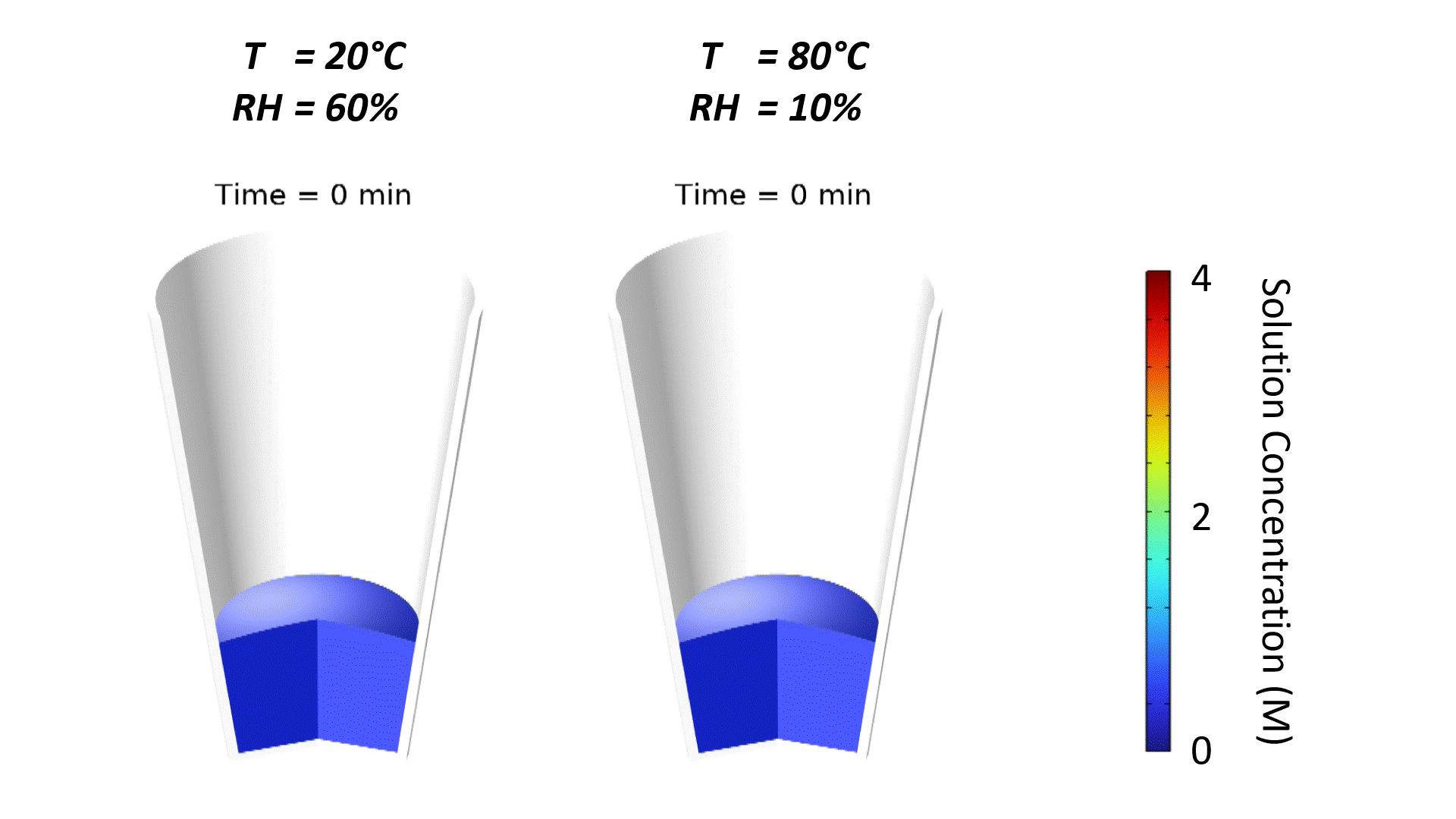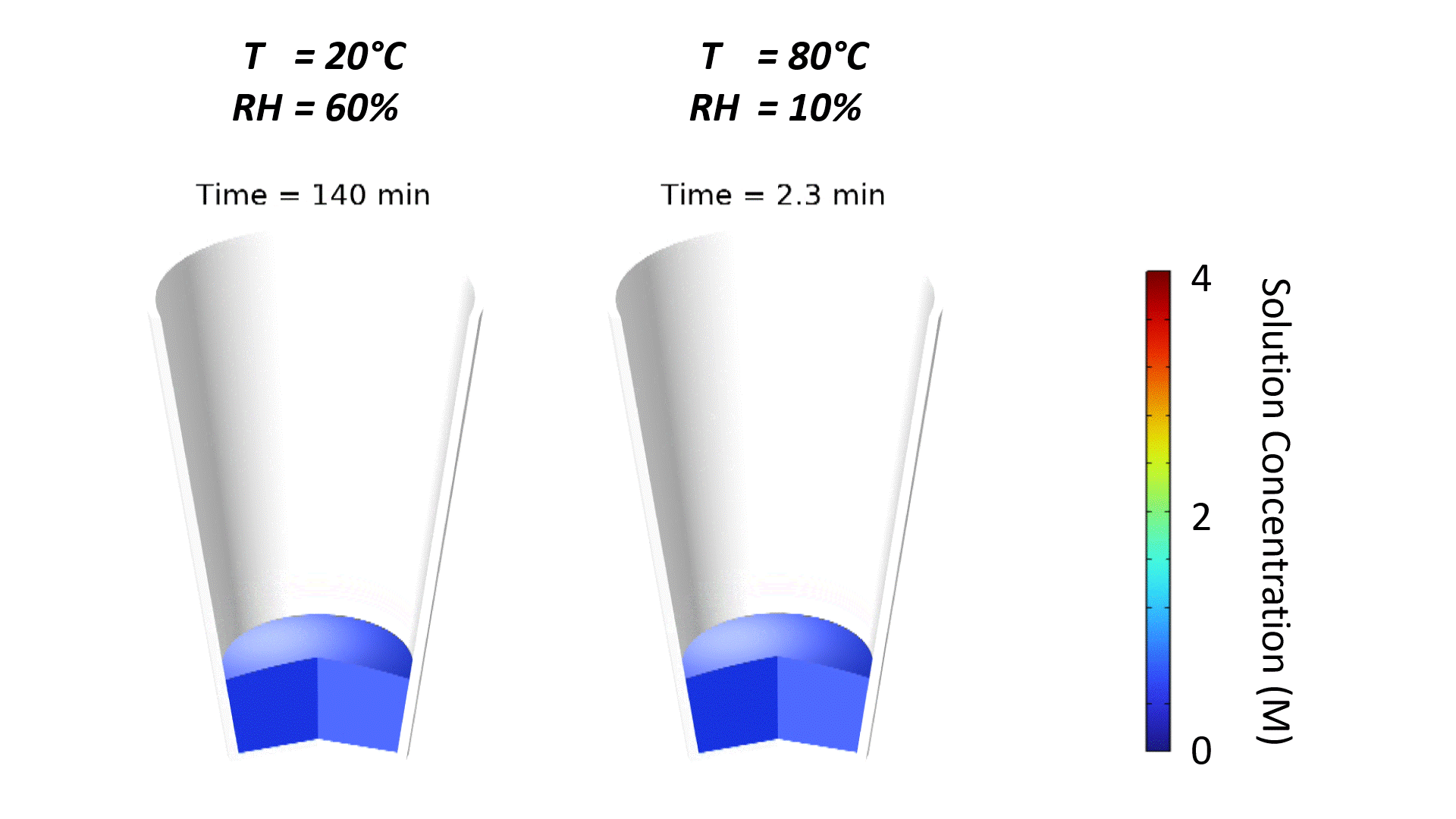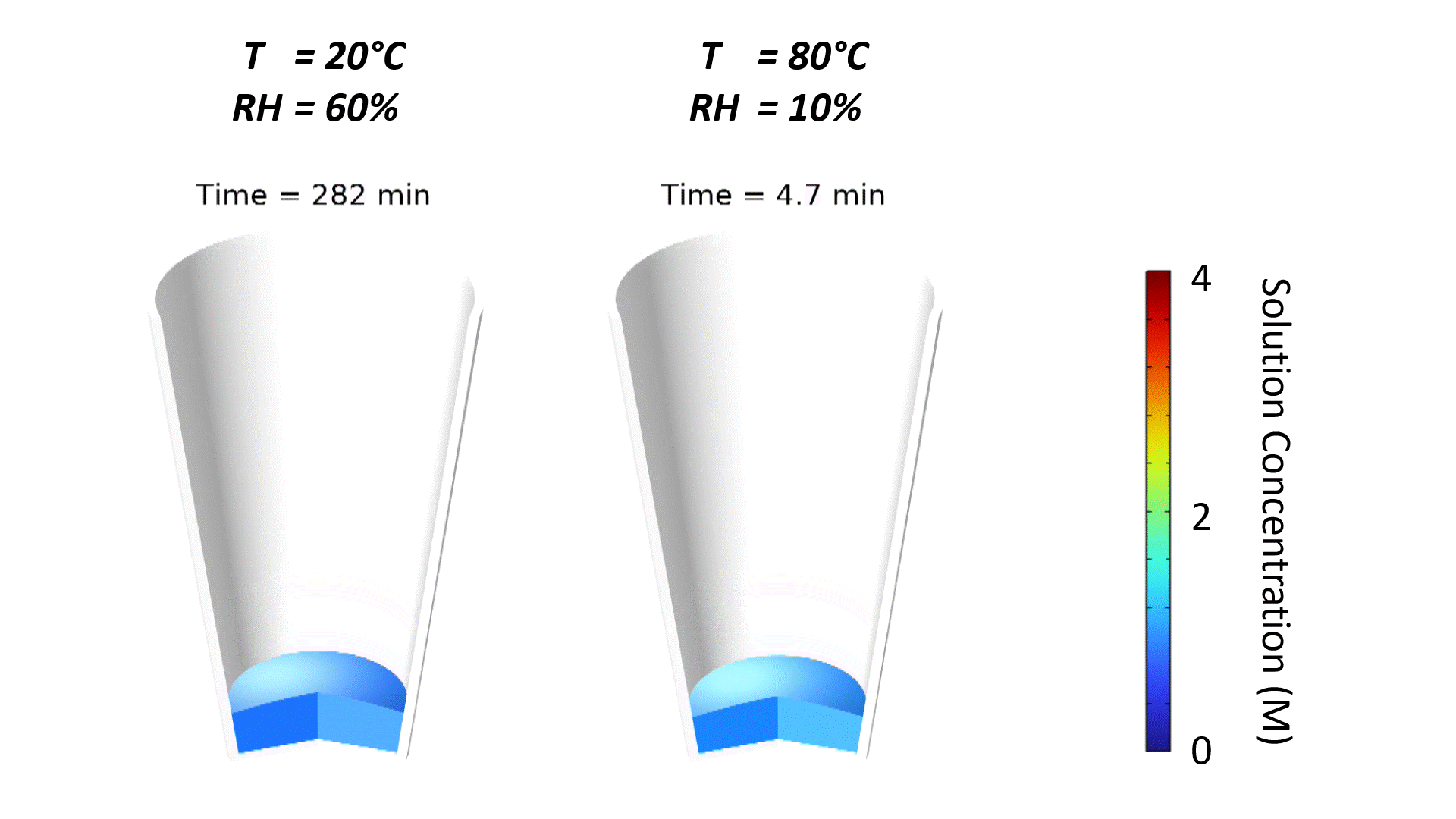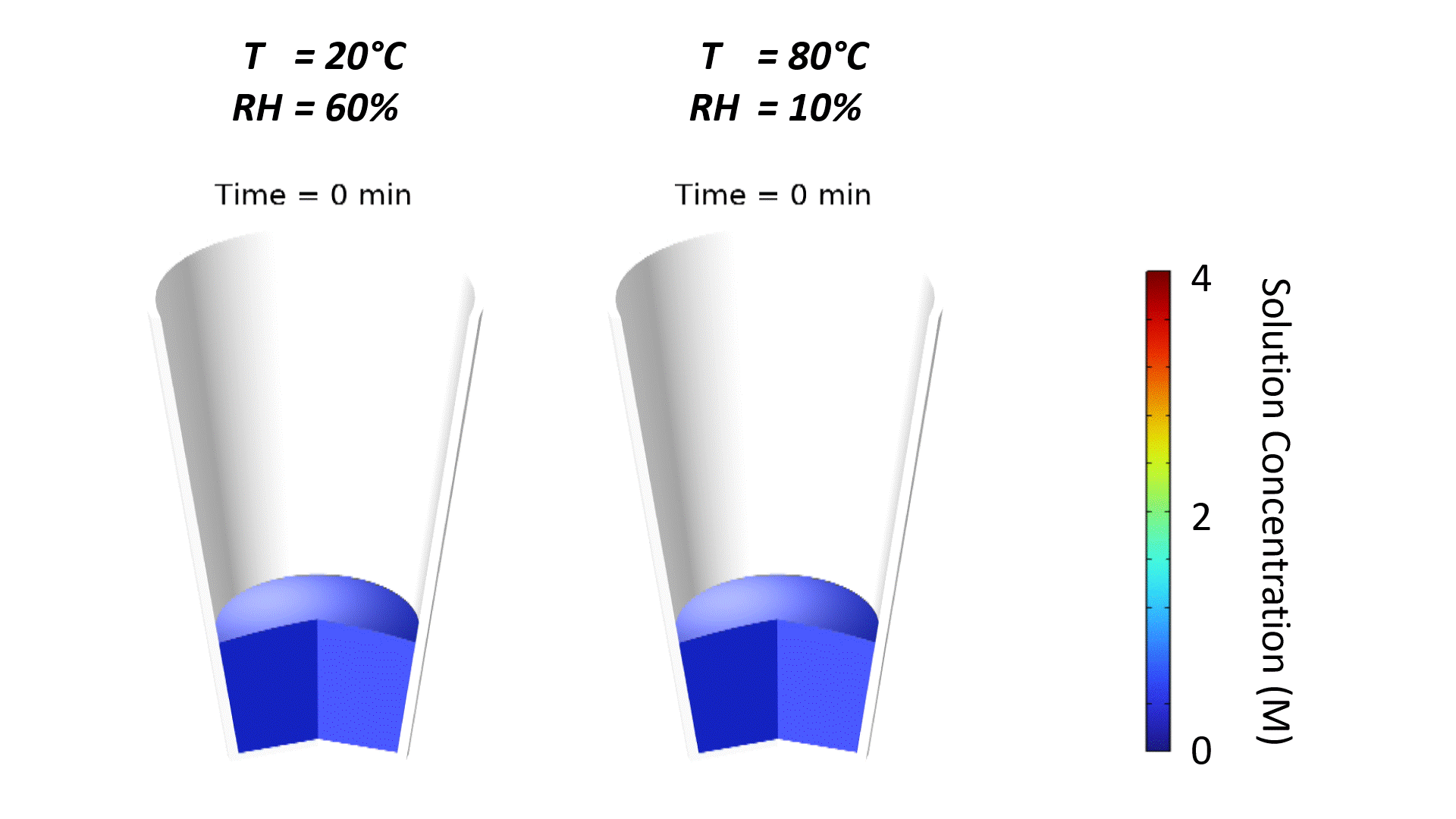
The strong effect of ambient conditions on dry-down is demonstrated in Figure 1. Increasing temperature and reducing relative humidity accelerates drying, which is why dehumidified air is often pumped into drying ovens. Note that the slight curvature of the free surface is due to the wetting properties of the microwell substrate, typically a plastic-like polystyrene. The contact angle of water on polystyrene is roughly 90°; since the well is tapered, the free surface curves to meet the substrate at a right angle.
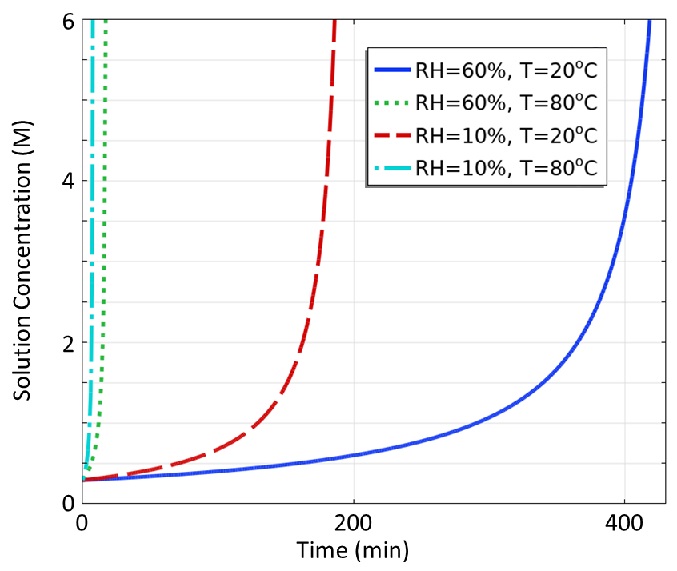
Oven temperature has the strongest impact on the evaporation rate. This is demonstrated in Figure 2 where simulated solution concentration is plotted vs. time for different process conditions. When the solution concentration reaches 6 M, the solution has been completely dried to a powder. Oven temperature has such a strong impact since the saturated vapor pressure and vapor diffusivity of water increase substantially with temperature, resulting in a higher evaporative mass flux at the free surface.
Conclusion
Veryst used COMSOL Multiphysics to simulate the effects of oven temperature and relative humidity on reagent dry-down in a microwell. Poor and differential air circulation (not simulated here) can lead to inhomogeneous drying across different microwells. The simulations shown here can be generalized to include air circulation within a drying oven to improve the uniformity of dried-down reagent characteristics.