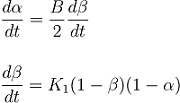Technical Challenge
A client wanted to reduce the thermal budget for an assembly process by reducing either the curing temperature or the curing time of a two-part epoxy-amine adhesive. The client also wanted a kinetic model to use for simulation of the curing process.
Veryst Solution
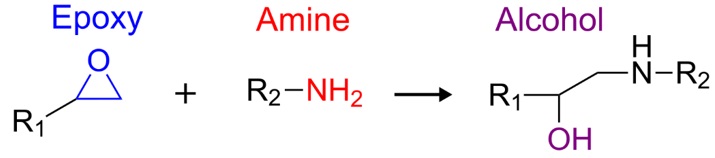
Veryst uses Fourier-transform infrared spectroscopy (FTIR) to investigate the evolution of the adhesive chemistry as the reaction proceeds. FTIR works by probing the infrared absorption properties of individual functional groups in organic materials. Epoxy-amine adhesives cure through the chemical reaction shown in Figure 1. As the cure proceeds, the number of epoxy groups and amine groups decreases, while the number of alcohol groups increases.
Figure 2 demonstrates how the FTIR spectrum evolves as the adhesive cures.
As the curing reaction proceeds, the FTIR absorbance bands for epoxy groups and amine groups decrease in intensity, while the absorbance bands for alcohol groups increase in intensity.
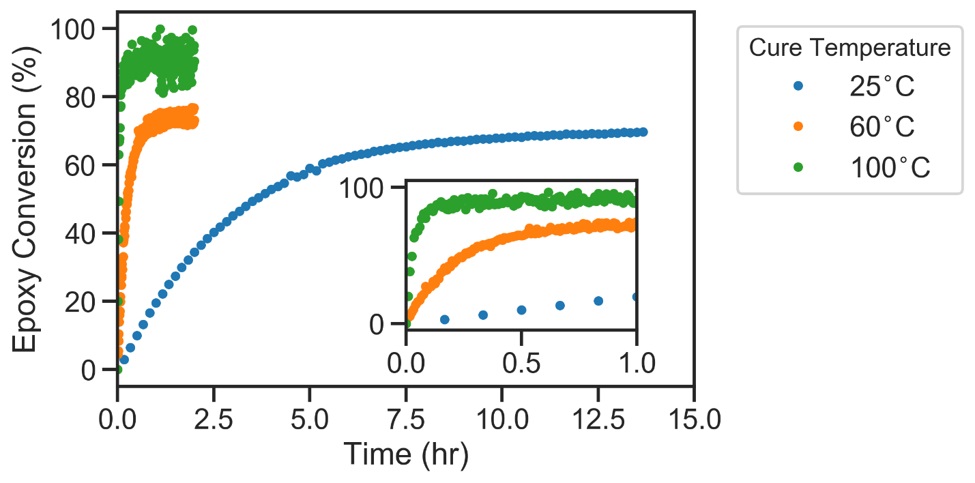
Figure 3 shows the epoxy group conversion at three different reaction temperatures.
The adhesive hardens as the degree of cure increases, and the reaction rate decreases dramatically. Because of the decrease in reaction rate, the adhesive only reaches approximately 70% cure at room temperature over 12 hours. In contrast, the adhesive reaches >90% cure in under 0.5 hours at 100°C.
Veryst also used the FTIR measurements to calibrate a kinetic model for the adhesive curing at room temperature:
Here, α is the % conversion of epoxy groups, β is the % conversion of amine groups, B is the ratio of initial concentrations of epoxy and amine groups, and K1 is a kinetic rate constant.
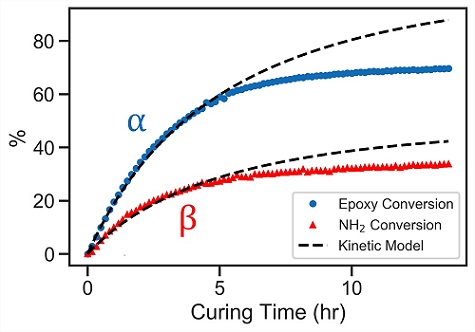
Figure 4 shows the values of α calculated from FTIR spectra and the predictions of the kinetic model.
The kinetic model accurately describes the curing kinetics in the early stages, up until approximately 50% of conversion. However, as the degree of cure increases, the adhesive hardens and the reaction rate decreases dramatically.
Nevertheless, the model accurately estimates both the epoxy and amine conversion rates for the first 5 hours of curing at room temperature.
This model could then be applied in simulations of the client’s assembly process to optimize the process variables. The result was a new set of process conditions that reduced the overall thermal budget without compromising the adhesive cure.