Technical Problem
Thermal ablation driven by an acoustic heat source has tremendous potential for the treatment of diseased tissue. In many applications, such as treating tumors in the liver, bulk removal of tissue is required. Thermal ablation by catheter-based acoustic applicators can reliably cause necrosis for a relatively large tissue volume by inducing coagulative necrosis.
Catheter-based acoustic ablation applicators can allow for more control over ablation than more common ablation techniques such as RF (radiofrequency) or HIFU (high intensity focused ultrasound) ablation. Acoustic ablation devices consisting of linear arrays of independently powered tubular transducers offer precise control of the heating pattern, allowing for the spatial power deposition to be altered both along the length of the applicator and through the axis for more accurate targeting. Catheter-based acoustic ablation devices have great potential for the treatment of cancer in regions that are surgically inoperable. However, there are challenges in developing a realistic ablation device model useful for design of these devices.
Veryst Solution
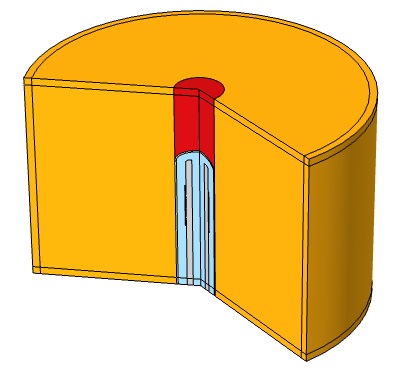
Veryst used COMSOL Multiphysics to simulate the propagation of acoustic waves through liver tissue for a 10-minute treatment period to understand the interaction between acoustics and heat transfer. Veryst designed a catheter ablation system with an array of piezoelectric acoustic transducers. We modeled the ablation device and tissue in COMSOL using a 2D-axisymmetric geometry (as shown in Figure 2).
The catheter system supporting the piezoelectric transducers was designed with a hollow central shaft to allow for a cooling fluid (25°C) to circulate through the device, keeping the temperature of the ablation device from reaching the desired ablation temperature in the tissue. We used a laminar flow model to simulate the circulation of the cooling fluid through the catheter system.
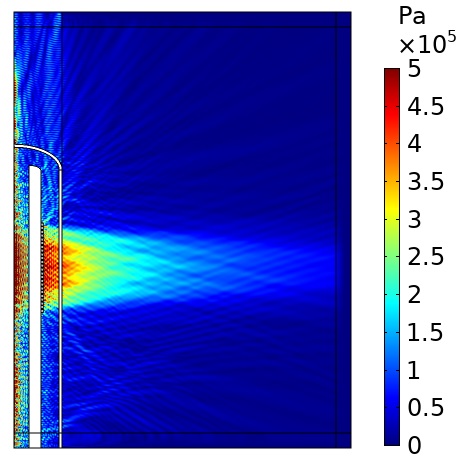
Using COMSOL, we modeled the acoustic pressure field from the ablation device using the power generated by a voltage difference on the piezoelectric transducers. By first running an eigenfrequency study to identify the frequencies with the desired mode shape of the transducers (displacement in the radial direction), we were able to identify a frequency that would give the desired acoustic pressure waveform (see Figure 3).
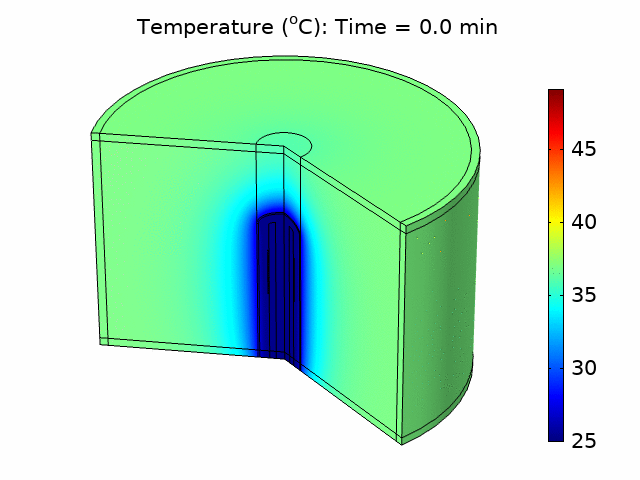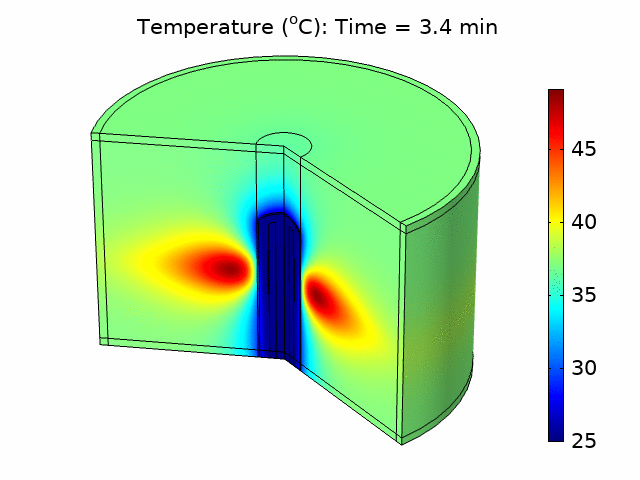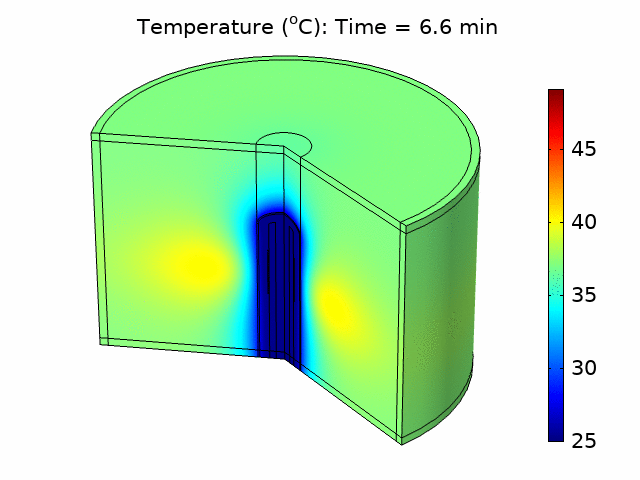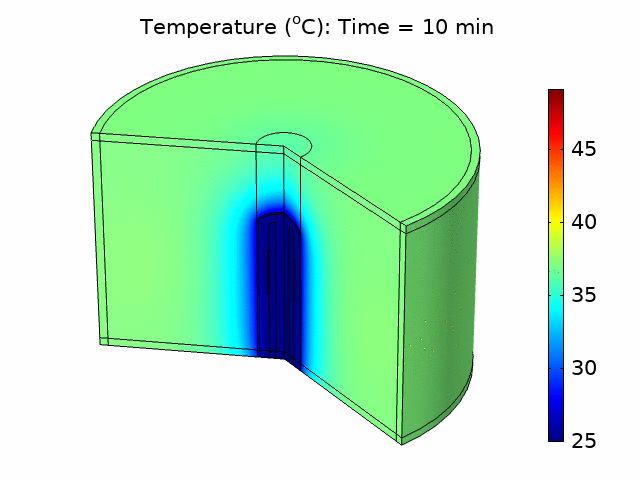
We used the plane wave power deposition density from the acoustic pressure study as a heat source in a bioheat transfer study in order to simulate heat transfer in the tissue. The resulting temperature distribution was caused by heating the tissue for 6 minutes, followed by 4 minutes of cooling with the power source turned off. The acoustic heat source raised the temperature in the tissue to a maximum value of 49°C over the 10-minute treatment period, taking into account blood perfusion (see the animation in Figure 1).
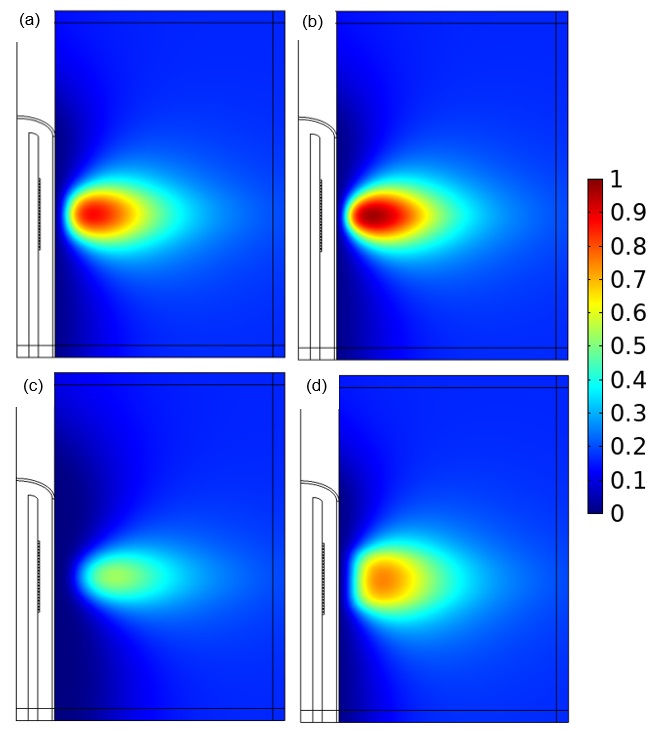
Veryst evaluated several changes to parameters in the ablation device, all of which affected the fraction of damaged tissue. The fraction of damaged tissue (with a value of 1 indicating necrosis) was evaluated using an Arrhenius kinetics damage integral.
Compared to the original design (a), the damage varied based on the number of active transducers used (b), the temperature of the cooling fluid (c), and the distribution of power in the transducers (d), as shown in Figure 4.
Conclusion
Using a COMSOL Multiphysics simulation to model bioheat transfer and thermal damage, Veryst was able to design a catheter-based acoustic ablation device with an array of piezoelectric acoustic transducers that could induce tissue necrosis using an acoustic heat source.