Driving Innovation in Combination Products with Multiphysics Simulation and Specialized Testing
Free, One-hour Webinar
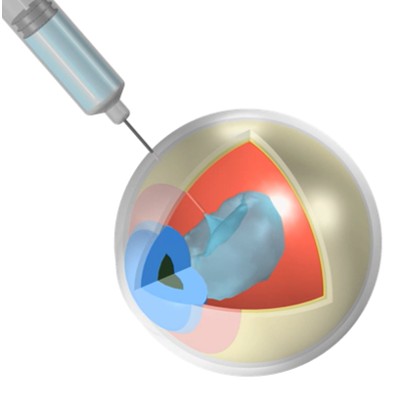
Combination products face unique obstacles in design, testing, and approval due to the complex interplay between drug and device. Advanced biologics bring challenges with stability, delivery complexity, and material compatibility that require development strategies beyond traditional approaches.
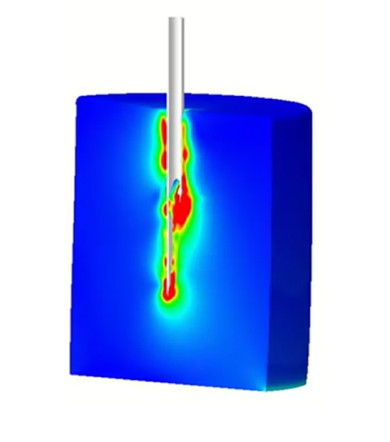
Traditional development, reliant on multiple prototypes and separate domain analysis, can be slow and costly. This webinar demonstrates how Veryst Engineering leverages deep subject matter expertise in mechanical engineering, materials science, chemical engineering, and physics with advanced multiphysics simulation and targeted testing to overcome these development hurdles.
By combining expertise with cutting-edge simulation tools to model fluid dynamics, structural mechanics, and species transport, teams can accurately predict behavior and performance from manufacturing to shipping to clinical use, optimize designs early, and mitigate risk. Simulation models, validated by focused lab tests, provide robust guidance and reliable decision-making across the development cycle.
Attendees will learn how this integrated approach accelerates development, reduces costly prototyping, and improves product safety by exposing potential issues early. Case studies will showcase how Veryst’s simulation-driven process helps clients innovate, maintain aggressive development timelines, and meet regulatory demands for even the most complex combination products and biologics.
Details
Course Instructors
Dr. Sean Teller is a Principal at Veryst Engineering. Dr. Teller has extensive experience in testing and modeling the mechanical behavior of materials, combination products, and medical devices. His expertise includes measuring the response of materials and products, simulating product and process performance, and failure analysis. Dr. Teller earned his Ph.D. from Brown University in Solid Mechanics, with research on high frequency, nonlinear viscoelasticity.
Registration
The February 19, 2026 Driving Innovation in Combination Products with Multiphysics Simulation and Specialized Testing web-based course is free, but registration is required and class size is limited.
Register at: https://events.teams.microsoft.com/event/82a045bb-267f-4692-970f-05e40e78ce91@79d1a68e-dd8f-4002-8514-fb1316d42162
If you have any questions, please email us at seminars@veryst.com
Deadline for registration is: Wednesday, February 18, 2026.
Cancellation Policy
Veryst reserves the right to reject registrations and to cancel a webinar based on class size.
Important Information
* You will receive an email confirmation once you have completed your registration.
* You will receive an email with login information the day before the webinar.
* If you don't see those emails, please check your junk or spam folder.
Dr. Matthew Hancock is a Principal at Veryst Engineering. Dr. Hancock has extensive experience in fluid mechanics and species transport related to product design and performance, with core areas including microfluidics, surface tension and wetting, and mixing. Within biopharma, MedTech, and combination products, he and his team consult on a range of topics including autoinjector performance, stopper lubrication, vial filling, lyophilization, drug product stability, jamming, and drug delivery. Dr. Hancock earned his PhD in environmental fluid mechanics from MIT. Prior to joining Veryst, he worked with the Broad Institute, the Wyss Institute, Brigham and Women’s Hospital, Harvard Medical School, and the Department of Applied Mathematics at MIT.