Veryst has deep expertise in consulting to the medical device industry, with experience that covers all phases of the product development process as well as failure analysis.
Manufacturing and Design
We work with medical device firms on design and manufacturing both to solve problems and accelerate development. We use advanced engineering tools that include applied finite element analysis, fabrication and joining technologies, material selection, and testing methodologies.
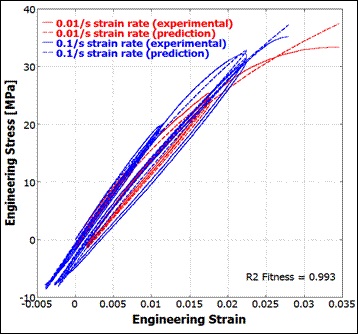
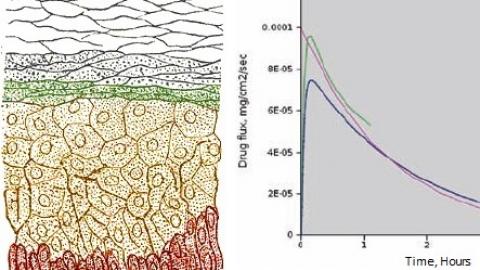
Our work ranges from developing new constitutive models that capture absorbable polymers' viscoelasticity to assisting with the development of MEMS medical devices; from modeling the transdermal drug delivery process to developing applications ("apps") that simulate RF tissue ablation.
Veryst offers unique expertise in advanced finite element analysis (FEA) of medical devices and implants. We use a combination of commercial finite element codes (Abaqus, ANSYS, COMSOL Multiphysics, and PolyUMod®—a software program originally developed by Veryst and now available through PolymerFEM.com).
Regulatory Support
Clients have used our analyses from early stage concept development through regulatory submissions (FDA 510k and PMA and CE Mark). We have provided finite element analysis support for Class I, II, and III device regulatory documents, and helped design validation experiments for fatigue and strength for FDA (U.S. Food and Drug Administration) submissions. We have also worked with firms in preparing responses associated with recall actions on topics of fatigue, contamination, strength, creep, and long-term testing.
Failure Analysis
Veryst’s world-leading capabilities in design, testing, manufacturing, and failure analysis also make us uniquely suited to investigate the failure of medical devices during development as well as post-production issues and root cause analysis.
Class III Devices
Implanted Devices
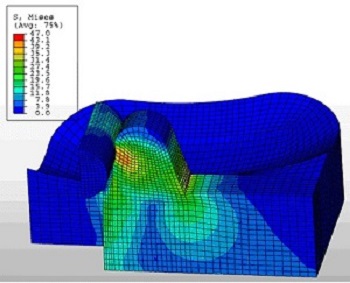
Veryst has used its expertise in nonlinear material behavior and large deformation simulation to assist medical device companies in the development of implanted devices such as stents, orthopedic implants, meshes, and fixation devices.
Vascular Implants
Veryst’s material modeling and FEA capabilities are used in the design and development of vascular implant systems, such as cardiovascular stents, valves, and grafts. Our advanced capabilities provide valuable information allowing for the optimization of designs early in the design process. Veryst is well-equipped to consider important advanced material behaviors, such as:
- Nonlinear viscoplasticity
- Shape-memory material behavior
- Material anisotropy due to manufacturing processes
- Effects of fatigue due to pulsatile loading
- Time-dependent material degradation of absorbable material
Orthopedic Implants
Veryst’s FEA capabilities are used in the design of orthopedic implants, such as hip and knee joint replacements. Advanced material models for ultra-high molecular weight polyethylene (UHMWPE), developed by engineers at Veryst, are used to analyze important load-bearing components and accurately evaluate the performance of new designs.
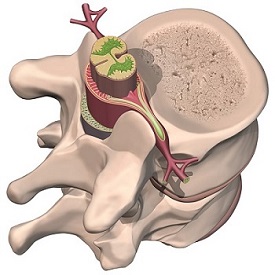
Spinal Implants
Our FE analyses are applicable in the design and failure investigation of spinal implant systems, including spinal fixation systems and advanced artificial disc or disc repair systems.
Advanced Biomaterials
Veryst’s advanced FE capabilities make us uniquely suited to assist in the development of advanced biomaterials, including:
- Synthetic and natural scaffold materials for tissue generation and wound healing applications
- Advanced medical adhesives and cements
- Materials for absorbable medical fasteners (sutures, screws, plates etc.)
Class II Devices
Veryst has supported medical devices companies in the design, failure analysis, and regulatory submission of numerous devices, including:
Catheters
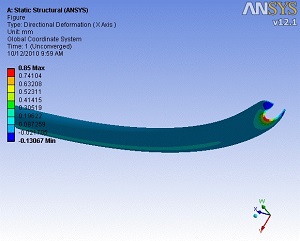
Catheters kink when passed around corners or tight radii within the body. Veryst’s FE analysis expertise has been used to complement a test program to investigate the factors contributing to catheter kinking.
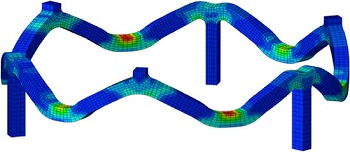
Patient Lifts and Supports
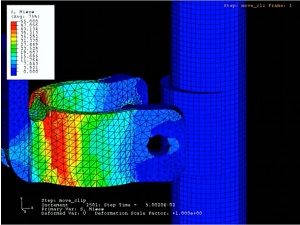
Veryst uses its analysis capabilities to investigate the design and reliability of patient lift and support systems. Veryst uses finite element analysis to assist in the design of critical components, frequently of systems comprising a wide variety of engineering materials including steel, aluminum, polymers, and composites.
Medical Devices Incorporating Fluid Flow
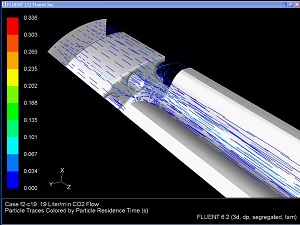
Veryst uses its multiphysics analysis capabilities to analyze combined thermal-fluid devices such as the gas humidification devices used with insufflation equipment.